Web Content Display Web Content Display
Volatile Discharge
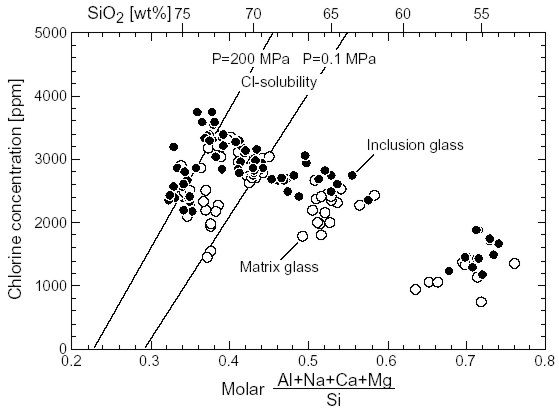
Determination Concentrations of S, Cl and F in inclusion and matrix glasses were determined by EMP. Inclusion glass is assumed to preserve the volatile concentration prior to degassing whereas matrix glass is considered to have degassed during equilibration to atmospheric pressure. The concentration differences (dS, dCl, dF = inclusion - matrix) should then yield the degassed volatile fractions. We have analyzed melt inclusions not containing exsolved fluid inclusions although fluid inclusions may have been removed during thin section preparation. A separate fluid phase may also have existed in the magma at the time of melt inclusion trapping. Our estimates of degassed volatile fractions are thus minimum estimates. There is some scatter in the data for each sample, reflecting variable trapping times and possible leakage of inclusions, variable adjustment to low pressure in the matrix, analytical uncertainties and possibly other factors. We have therefore determined average, maximum and minimum -values of degassed volatile fractions for each sample. Volatile concentrations Sulfur and chlorine concentrations always drop from inclusion to matrix glass indicating degassing. Fluorine concentrations, however, mostly remain constant or even increase toward the matrix, suggesting there was little to no fluorine degassing in agreement with experimentally determined DF fluid/melt<1 at low F-concentrations. While fluorine solubility in silicic melt is practically unlimited, chlorine solubility depends on melt chemistry and pressure. Chlorine thus may degas either by exceeding the solubility limit or by partitioning into an aqueous fluid phase at DCl fluid/melt>>1.Comparison of measured Cl-concentrations in glass inclusions with solubility limits from Webster et al. (1999) show that mafic to intermediate melts remained Cl-undersaturated (Fig. 1) and Cl-degassing in these magmas occurred exclusively by partitioning into the fluid phase from exsolving water. Decompression of rhyolitic melts, however, shifted Cl-concentrations above the solubility limit and hence Cl-degassing would have occurred even if no water had exsolved (Fig. 1).
Fig. 1: Measured chlorine concentrations in inclusion (filled symbols) and matrix glasses (open symbols) as a function of glass composition to be compared to Cl-solubility limits at 200 MPa atmospheric and pressure after Webster et al. (1999). Since water did exsolve, Cl-degassing operated by both mechanisms in the rhyolitic melts. Clconcentrations in rhyolitic matrix glasses are mostly not reduced to the 1-atm solubility suggesting the both processes of chlorine release were limited by Cl-diffusion rates too slow to maintain equilibrium during rapid magma ascent, eruption and quenching. While using average concentration and -values to characterize the volatile budgets of the various eruptions, detailed analyses show that degassing behavior during a single eruption can vary significantly. For example, the basaltic plinian Fontana eruption evolved from an initial highly unsteady discharge of the most primitive magma through a main steady plinian phase of constant basaltic-andesite composition to a terminal unsteady subplinian phase. The degassed fraction of sulfur (S) remained approximately constant throughout the eruption (Fig. 2). The initial chlorine concentration (in melt inclusions) was constant except for a lower concentration in the more mafic initial phase; the degassed chlorine fraction (Cl), however, changed significantly with time in response to variable chlorine loss from the matrix melt (Fig. 2). This observation may be explained by variably efficient partitioning of chlorine into an aqueous vapor phase and we are presently performing H2O-analyses of inclusion and matrix glasses to pursue this theory.
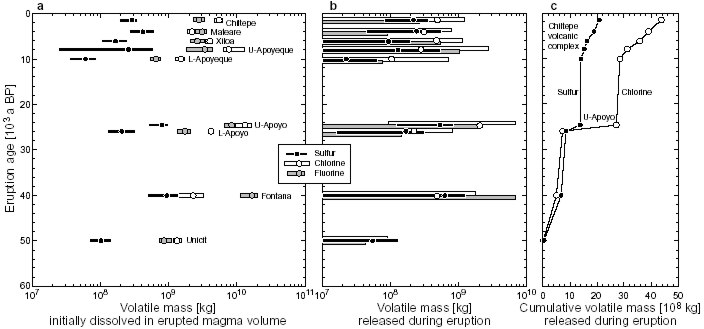
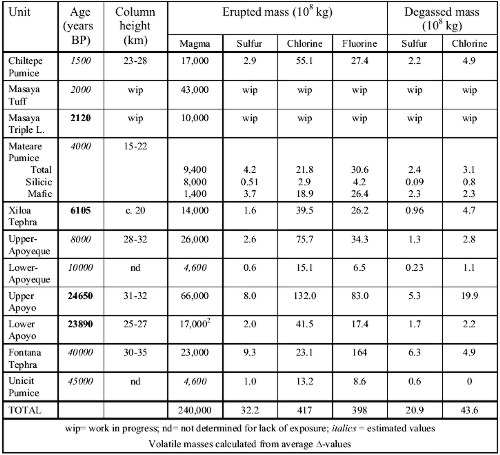
Fig. 2: The degassed fraction of sulfur (S) varies little across the stratigraphic sequence of the basaltic plinian Fontana Tephra. The gassed fraction of chlorine (Cl), however, changes significantly due to variably incomplete matrix degassing. Volatile masses Volatile masses injected into the atmosphere during the highly explosive eruptions (Fig. 3b) are obtained by multiplying the erupted magma mass by the degassed volatile fraction. Similarly, the total volatile mass transported to the surface (Fig. 3a) is the product of erupted magma mass times the fraction of volatiles in the melt inclusions. Calculated data are compiled in Table 1. As noted above, minimum and average values of F<0 and only maximum values of F>0 suggest there was little if any fluorine degassing in all eruptions analyzed. Positive average values of S and Cl yield masses of order 108 kg of both these volatiles released into the atmosphere during almost each eruption (Fig. 3b). The petrologic data suggest that chlorine release almost always exceeded sulfur release, a result that is based on the assumption that no sulfur-rich fluid phase already existed at the time of inclusion trapping. The largest-magnitude Upper-Apoyo eruption also discharged the by far largest mass of chlorine (2*109 kg) into the stratosphere, producing a marked jump in the cumulative gas-release trend (Fig. 3c).
Fig. 3: (a) Initially dissolved masses of S, Cl and F transported to the surface with the erupted magma mass of each eruption. Dots are data based on average concentrations, bars cover data from minimum to maximum observed concentrations. Note halogen-rich nature of all magmas, although S-content may be underestimated if a S-rich fluid phase existed in the reservoirs. Undetermined eruption ages are estimated from geologic context as shown in Table C4-2. (b) The respective volatile masses released into the stratosphere during eruptions. Note that for fluorine only maximum -values are positive while average and minimum values are negative, suggesting there was little to no F-degassing despite the relatively F-rich nature of the magmas. (c) Cumulative sulfur and chlorine mass release into the atmosphere. Note jump due to the large-magnitude, Cl-rich Upper-Apoyo eruption and increasing divergence of the curves in favor of chlorine during activity of the Chiltepe Volcanic Complex. Another feature of the cumulative trends is that the rates of sulfur and chlorine mass injection into the atmosphere increased over the past 10,000 years of frequent activity at the Chiltepe Volcanic Complex, with chlorine accumulating faster than sulfur (Fig.3c). Fig. 3a shows the volatile masses transported to the surface by the erupted magmas, which are typically on the order of 108 kg for sulfur and 109 kg for chlorine and fluorine. Comparison with degassed masses (based on average -values) shows that at least 50-80% of the initially dissolved sulfur but only 5-20% of chlorine and almost no fluorine had actually degassed during the eruptions. The vast majority of the magmatic halogens thus is not released into the atmosphere but remains trapped in the ash and is ultimately transported into the Pacific ocean where it may be subject to chemical exchange with pore- and seawater or re-enter the subduction cycle. First SyXRF analyses of glasses from the basaltic Fontana plinian fallout suggest that this eruption released 3*106 kg bromine, which is by far more climatically active than chlorine. Volatile fluxes Our estimates of the cumulative flux of volatiles from highly explosive arc volcano eruptions are very preliminary at present mainly for two reasons: (1) the time window studied needs to be expanded and more eruption ages need to be determined, and (2) the arc section length studied needs to be extended since present results are essentially from only three volcano-magma systems of quite different compositional and eruptive history, so that the significance of any averaged results remains speculative. For the arc segment studied and assuming a 40,000 years time span, we obtain time-averaged fluxes of 8.1*104 kg/a S, 1*106 kg/a Cl and 1*106 kg/a F to the surface, of which 5.2*104 kg/a S, 1.1*105 kg/a Cl and nearly no F are injected into the stratosphere. Considering the past 10,000 years only, fluxes to the surface are 1.2*105 kg/a S, 2.1*106 kg/a Cl and 1.3*106 kg/a F, and fluxes into stratosphere are 7.1*104 kg/a S and 1.7*105 kg/a Cl. All these flux values might be at least doubled if intrusive magma volumes were also considered. Table 1: Summary of eruption and volatile data
The magma flux from mantle of 7.8*109 kg/a estimated by subproject C2 compares to 1.2*109 kg/a (past 10,000 yrs) to 5.9*108 kg/a (past 40,000 yrs) magma flux to the surface by highly explosive eruptions; considering also intrusive volumes of the magma systems we have studied, the implication would be that some 8-15% of mantle-derived magmas end up in highly explosive eruptions. A similar range of 8-26% is obtained from comparing the chlorine flux of 3.9*106 to 1.3*107 kg/a from the mantle estimated by C2 with our estimates. Hence, the ratio of chlorine fluxes agrees well with the ratio of magma fluxes. In contrast, the magmatic sulfur flux of 7.8*106 to 1.7*107 kg/a from the mantle wedge into the crust as determined by subproject C2 is one to two orders of magnitude larger than our sulfur flux estimates. This discrepancy suggests that separate sulfur-rich fluid phases (which remain undetected by our melt inclusion analyses) exsolved during magmatic differentiation and mainly governed the sulfur flux. Such sulfurous fluids may already have formed from the mafic magmas since the sulfur flux of 3*108 kg/a from permanent degassing of active Nicaraguan volcanoes as estimated by subproject C3 by far exceeds the estimated flux from the mantle. The fact that also the chlorine flux of 1*108 kg/a from permanent degassing is much higher than the other estimates suggests that part of the problem may lie in our ignorance of the intrusive magma volumes involved in the arc volcanic system. Additional uncertainties arise from the different spatial and temporal scales and error margins involved in the flux estimates. |
 Events Events
Kieler Wissenschaftler fühlen den 'Puls der Erde' Wie funktioniert die Recyclingmaschine der Erde?Nach elf Jahren endet der Kieler Sonderforschungsbereich 574 zu Subduktionszonen Final colloquium of SFB 574 Teilprojekt ÖffentlichkeitsarbeitMEERESFORSCHUNG FÜR MICH UND DICH |
|
©SFB574 // Wischhofstrasse 1-3 // D-24148 Kiel // T. +49 (0)431 600 1413 // elange [AT] geomar.de